
| Staff | Professor | Masaaki Sugiyama | Assistant Professor | Akio Kawaguchi |
|---|---|---|---|---|
| Associate Professor | Rintaro Inoue | Assistant Professor | Akiko Kita | |
| Assistant Professor | Masahiro Shimizu | Assistant Professor | Ken Morishima |
It is well known that a material structure and its dynamical character are deeply related. The interaction between the constituents determines the material structure and the dynamical character is a response of the structural interaction against the external disturbances. In the case of a functional material with a nano-scale structure, it is essential to reveal a mechanism of function to understand its dynamical character based on the structure. Along this line, this research group studies the static and dynamical structures of functional materials with nano-scale structures such as supercritical fluid, polymer aggregates, gel and protein. Main methods to measure nano-scale structure are scattering techniques with X-ray or neutron: especially, neutron scattering utilizing its ability identifying isotopes, proton and deuteron, is a very powerful tool to reveal a quaternary structure of protein. Recently, we revealed a state of PA28 (Proteasome Activator 28) in an aqueous solution: PA28 is a regulator protein of the 20S proteasome, which is protease for an ubiquitinated protein. Figure 1 shows the SANS analysis of the state of PA28 in an aqueous solution. The structural simulation well reproduces the experimental SANS profiles. In addition, we are developing a spectrometer and analyzing methods. Therefore, we are joining the TAIKAN project (SANS in J-PARC) and also developing a SANS and SAXS simulation with RMC algorism.
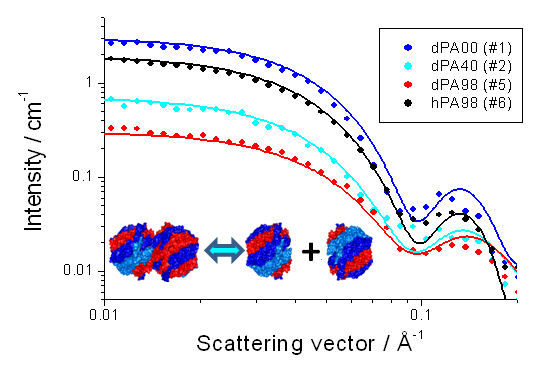
Figure 1: Structural model, SANS profiles. Circles denote experimental data and lines show the result of the simulation.